After your grids are prepared, you are now ready for cryo-EM imaging. All cryo-EM grids need to be screened in order to decide whether resources should be committed towards data collection. The ideal grid contains a rich distribution of monodisperse particles that is supported by a thin layer of vitreous ice. In practice, a number of common issues at the sample or grid level can prevent the justification of data collection. This chapter covers best practices in the screening process, starting from a procedural overview of how grids are loaded into the TEM to assessing whether a grid is ready for data acquisition.
Depending on your facility, your microscopes may use either a traditional side-entry or a more modern automatic grid loading system. Regardless of your system, the general principle underlying all grid loading procedures is the same: your vitrified grids must be carefully secured in place and transferred into the microscope while maintaining the grids at cryogenic temperatures.
The following videos provide an overview of a side-entry and autoloading systems. Remember that all tools that come in contact with your grid boxes and grids must first be chilled to liquid nitrogen temperatures, otherwise you risk warming up your grid! Due to the highly technical nature of these procedures and differences between the equipment shown in the videos and your facility, it is important to receive training from your facility manager and work under a supervised setting in order to gain familiarity with your setup.
Side-entry system
The side-entry holder and its accompanying workstation are first chilled and equilibrated at cryogenic temperatures. Connect your holder to a temperature probe to verify it is near the boiling point of liquid nitrogen (i.e., about -196°C). A grid box is carefully transferred into the workstation. The box lid is loosened and then a grid is placed onto the tip of the cryo-holder. A clip ring is then secured over the grid and the holder is covered with a shutter to protect the grid from exposure to the surrounding environment. Before inserting the holder into the microscope, check that the airlock has been pre-pumped to preserve the column vacuum.
Auto-loading system
The latest generation of cryo-TEMs use an auto-loading system that offers greater stability over traditional side-entry holders. Autoloaders require grids to be assembled in “auto-grid” cartridges before they are inserted into the microscope. Autogrids are assembled in dedicated workstations by seating cryo-EM grids over a clip ring and then locking the grid and clip ring together with a C-shaped spring, or C-clip. The autogrid is then carefully inserted into one of several slots within the autoloader cassette. Finally, the cassette is loaded into the TEM.
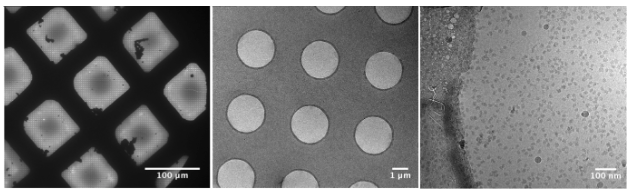
After the cryo-EM sample is successfully inserted into the TEM, the grid is ready for evaluation. During the screening phase, images are typically recorded at a range of magnifications to check for ice and particle quality.
Grid view: Low magnification views of the cryo-EM grid can be extremely informative about the general ice quality of the specimen. At this range, ice that is overly thick will block or obscure views of grid squares. Many vitrified grids will show a gradient of ice thickness.
Square view: Midrange magnification views are useful for confirming ice quality within grid squares. These images can reveal the presence of vitreous or crystalline ice, contamination, and variations in ice thickness.
Hole view: High magnification views are necessary to evaluate the particle itself. The perfect grid will have a rich distribution of mono-disperse particles throughout the hole. If this criterion is not met, then the optimization strategies described in Chapters 1 and 2 should be considered. Note that small particles may only be visible at high defocus levels.
Note that small particles may only be visible at high defocus levels.
Cryo-EM grids often exhibit a range of ice and particle quality. The following gallery contains many examples of real cryo-EM images that are categorized by common features.
The cryo-EM project workflow should be treated as an iterative process that requires going back-and-forth between sample purification, grid preparation, and grid screening. The content covered up to this point explains the most common strategies used to optimize samples and grids and to assess whether a grid is ready for data collection. Almost every sample will require some level of optimization, such as tuning the right concentration of sample or finding the ideal conditions for achieving the best ice. The only way to be sure that grids are suitable for data collection is to screen them using the guidelines discussed in this chapter. After you are convinced you have high-quality grids, you are ready to move on to the next chapter.